Ocean Acidification
Ocean acidification is defined as a decrease in ocean pH over decades or more that is caused primarily by uptake of carbon dioxide (CO2) from the atmosphere.
The concentration of atmospheric CO2 has increased dramatically since the Industrial Revolution, from around 280 parts per million (ppm) in preindustrial times to 424 ppm as of May 2024. This increase in atmospheric carbon dioxide (CO2) is absorbed by the ocean and leads to changes in the ocean’s carbonate chemistry, commonly referred to as ocean acidification.
Changes in Ocean Chemistry
When CO2 is absorbed by the ocean, chemical reactions occur. In particular, carbonic acid is formed and hydrogen ions are released; as a result, the pH of the ocean surface waters decreases, making them more acidic. When hydrogen ions are released in seawater, they combine with carbonate ions to form bicarbonate. This process lowers the carbonate ion concentration. The reduction of available carbonate ions is a problem for marine calcifiers, such as corals, crustaceans, and mollusks, who need the carbonate ions to build their shells and skeletons.
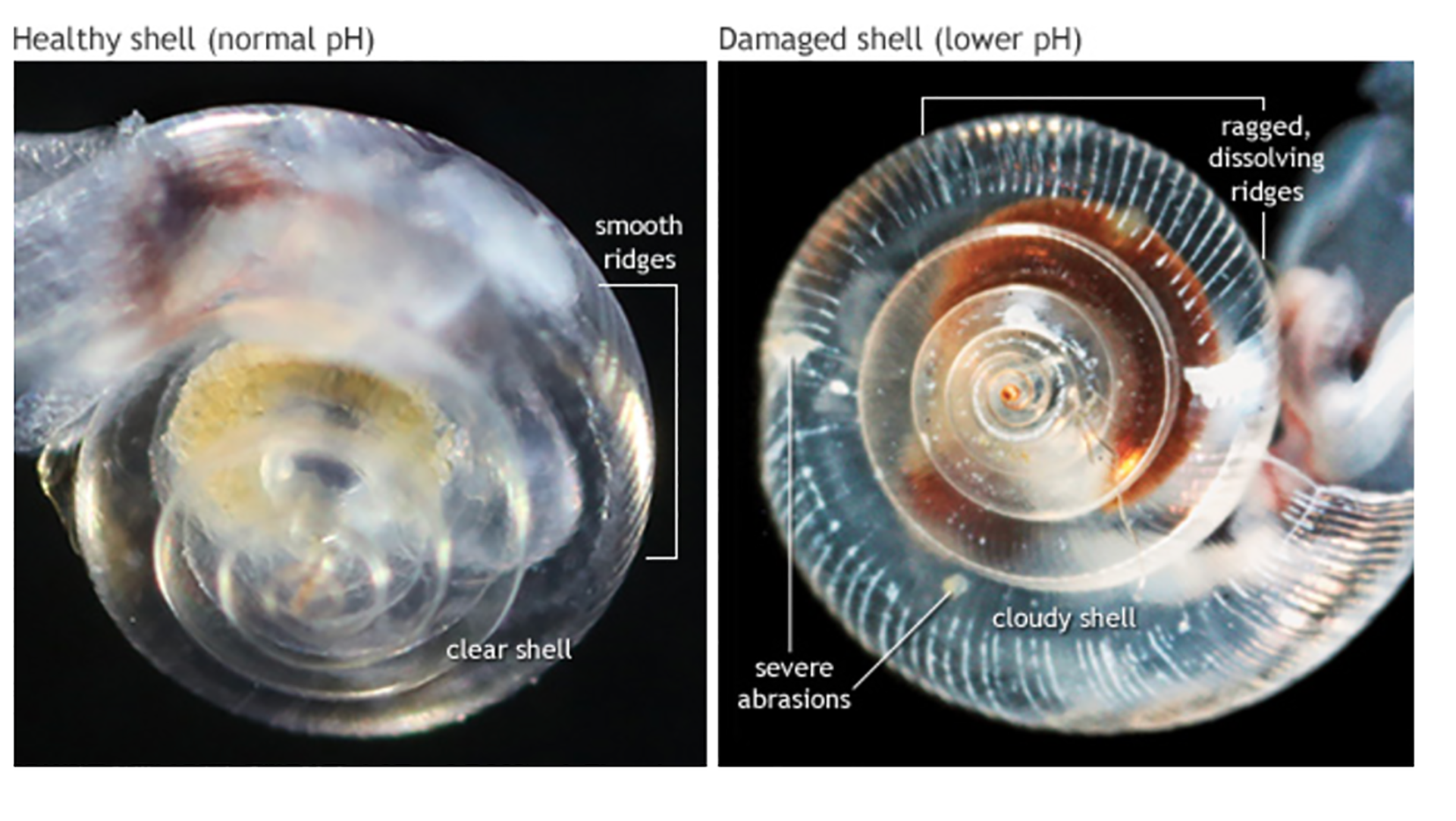
Example of the effects of ocean acidification on shells. The healthy shell on the left is transparent with smooth ridges; by contrast, the shell exposed to more acidic, corrosive water is cloudy, ragged, and marked with weak spots. Photo © National Oceanic and Atmospheric Administration
Impacts on Reef Building Corals
Since reef-building corals need carbonate to build their skeletons, decreasing carbonate ions will likely lead to weaker, more brittle coral skeletons and slower coral growth rates. This may cause coral reefs to erode faster than they can calcify, thus decreasing the ability of coral species to compete for space. Acidification also impacts the community of crustose coralline algae (CCA), critical to coral recruitment. Ultimately, direct and indirect impacts of acidification affect coral communities through changes in coral cover, and altered community composition and species diversity. ref
Socio-economic Impacts
Ocean acidification will decrease the abundance of commercially important shellfish species, such as clams, oysters, and sea urchins, which will affect the human communities that depend upon these resources for food and/or livelihoods. ref Decreases in calcification and substrate stability will also impact coral reef structural complexity, and their capacity to absorb wave energy, and mitigate coastal erosion and impacts from tropical storms.
Management Strategies
Currently, the best guidance for managing for ocean acidification involves prioritizing management towards protecting natural refugia and managing local stressors on reefs. Management strategies that protect these natural refugia from other stresses may help reefs cope with predicted changes in climate and ocean chemistry.
Management strategies to reduce the impacts of ocean acidification include:
- Design MPAs that consider OA – Include coral reef areas in a variety of ocean chemistry and oceanographic regimes (e.g., high and low pH and aragonite saturation state) in MPAs.
- Reduce threats that exacerbate ocean acidification conditions
- Explore and apply innovative interventions
- Reduce the effects of OA – Implementing national or global policies to drastically reduce global carbon emissions is the most critical step towards reducing the effects of ocean acidification.
